Iodine Derivatives
- Methyl Iodide
- Ethyl Iodide
- n-Butyl Iodide
- N-propyl Iodide
- 2-Ethylhexyl Iodide
- Diiodomethane
- Chloroiodomethane
- 5-Iodo-2-methylbenzoic Acid
- 2-chloro-5-iodobenzoic acid
- Methyl-2-Iodobenzoate
- 2-Iodobenzoic Acid
- 3,5-Diiodosalicylic Acid
- N-Iodosuccinimide
- Tetrabutylammonium Iodide
- Iodine Monochloride
- 5-Iodoanthranilic acid
- Iodoform
- Potassium iodide
- Sodium Iodide
- Ammonium Iodide
- Cuprous Iodide
- Zinc Iodide
- Sodium Metaperiodate
- Potassium Iodate
- Calcium Iodate
- Iodic acid
- Periodic acid
- Hydriodic Acid
- Iodobenzene Diacetate
- Dess Martin Periodinane
- 1,4-Diiodobenzene
- 4-Bromoiodobenzene
- 4-Chloroiodobenzene
- Iodobenzene
- 4-Iodoaniline
- 4-Iodotoluene
- 4-Iodoanisole
- 4-Iodophenol
- 4,4'-Diiodobiphenyl
- Trimethylsulfoxonium Iodide
Methyl Iodide
Methyl iodide is also called Iodomethane having Molecular formula CH3I, Mol. Wt. 141.94 g/mol. It is an important halo alkane derivative of Methane, in which one atom of hydrogen in Methane is replaced by one atom of Iodine. Methyl Iodide is an Organo iodine Compound that contains one Carbon – Iodine bond. Iodomethane contains Methyl group, with formula CH3 (also called Me). In Methyl Iodide it exists as Methylium cation ( CH3+) which is obtained by protonation of Methanol. Methyl iodide is highly reactive, the Methylium cation acts as a strong electrophillic Methylating agent, while the Iodide ion is a powerful nucleophile and is a good leaving group. Methyl iodide is a dense, colorless, volatile liquid turns brown on exposure to light due to liberation of Iodine. Methyl Iodide has a very low boiling point (B.P. 41-43 °c), Sp. Gr. at 20 °c: 2.27 – 2.28gm/cc. Iodomethane is slightly soluble in water, miscible with Alcohol, Ether. Being an organo iodide compound, Methyl Iodide is sensitive to light and therefore needs to be stored in dark amber colored bottles to prevent its degradation by light. Iodomethane is stabilized by Copper or Silver wire.
Methyl iodide is prepared by any one of the following methods :
- By the reaction of Iodine on Mixture of Methanol and Red phosphorous.
- By the reaction of Dimethyl Sulphate with Potassium Iodide
- By the reaction of Methanol with Potassium iodide catalyzed by Acid.
It finds its main applications :
- In the Organic synthesis as a Methylating agent for Methylation of Carboxylic acids ,Phenols.
- Methyl Iodide is used in the preparation of Tetra Methyl Ammonium Hydroxide
- Iodomethane is used in the preparation of Methyl Magnessium Iodide (Grignard reagent).
- as a pesticide(v) For Preplant soil treatment in the fields of strawberries , peppers, tomatoes.
| Product | Methyl Iodide (Iodo methane) |
| Structural formula | 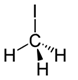 |
| CAS No. | [74-88-4] |
| Molecular formula | CH3I |
| Molecular weight | 141.94 |
| Description | Clear colorless liquid |
| Density | Between 2.270 and 2.285 g/ml. |
| Water | Max. 0.10 % |
| Assay | Min. 99.0 %. |