Selenium Derivatives
Selenium Dioxide
Selenium Dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of Selenium Dioxide. Selenium Dioxide is prepared by oxidation of Selenium by burning in air, nitric acid or by reaction with Hydrogen Peroxide, but perhaps the most convenient preparation is by the dehydration of Selenous Acid.
| Product | Selenium Dioxide |
| Structural formula | 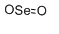 |
| CAS No. | [7446-08-4] |
| Molecular formula | SeO2 |
| Molecular weight | 110.96 |
| Description | white hygroscopic powder. |
| Iron | Max. 0.005 %. |
| Heavy metals | Max. 0.01% . |
| Assay | Min. 99.0%. |