Selenium Derivatives
- Methyl Iodide
- Ethyl Iodide
- n-Butyl Iodide
- N-propyl Iodide
- 2-Ethylhexyl Iodide
- Diiodomethane
- Chloroiodomethane
- 5-Iodo-2-methylbenzoic Acid
- 2-chloro-5-iodobenzoic acid
- Methyl-2-Iodobenzoate
- 2-Iodobenzoic Acid
- 3,5-Diiodosalicylic Acid
- N-Iodosuccinimide
- Tetrabutylammonium Iodide
- Iodine Monochloride
- 5-Iodoanthranilic acid
- Iodoform
- Potassium iodide
- Sodium Iodide
- Ammonium Iodide
- Cuprous Iodide
- Zinc Iodide
- Sodium Metaperiodate
- Potassium Iodate
- Calcium Iodate
- Iodic acid
- Periodic acid
- Hydriodic Acid
- Iodobenzene Diacetate
- Dess Martin Periodinane
- 1,4-Diiodobenzene
- 4-Bromoiodobenzene
- 4-Chloroiodobenzene
- Iodobenzene
- 4-Iodoaniline
- 4-Iodotoluene
- 4-Iodoanisole
- 4-Iodophenol
- 4,4'-Diiodobiphenyl
- Trimethylsulfoxonium Iodide
Ammonium Iodide
Ammonium Iodide is ammonium salt of Hydriodic Acid, available as white crystalline power. It is highly soluble in water and also in ethanol. Ammonium Iodide has molecular formula NH4I. On exposure to atmosphere it turns yellow due to liberation of Iodine. Ammonium Iodide prepared by the action of Ammonia or its salt on Hydriodic Acid. It has wide range of applications in chemical industries.
| Product | Ammonium Iodide |
| Structural formula | 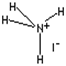 |
| CAS No. | [12027-06-4] |
| Molecular formula | NH4I |
| Molecular weight | 144.94 g/mol |
| Description | White hygroscopic powder. |
| Heavy metals | Max. 0.01 % |
| Chloride/bromide (as Cl) | Max. 0.05 % |
| Loss on drying | Max. 0.50 % |
| Sulphate | Max. 0.05 %. |
| Iron | Max. 0.01 % |
| Assay | Min. 99.0 % |