Selenium Derivatives
- Methyl Iodide
- Ethyl Iodide
- n-Butyl Iodide
- N-propyl Iodide
- 2-Ethylhexyl Iodide
- Diiodomethane
- Chloroiodomethane
- 5-Iodo-2-methylbenzoic Acid
- 2-chloro-5-iodobenzoic acid
- Methyl-2-Iodobenzoate
- 2-Iodobenzoic Acid
- 3,5-Diiodosalicylic Acid
- N-Iodosuccinimide
- Tetrabutylammonium Iodide
- Iodine Monochloride
- 5-Iodoanthranilic acid
- Iodoform
- Potassium iodide
- Sodium Iodide
- Ammonium Iodide
- Cuprous Iodide
- Zinc Iodide
- Sodium Metaperiodate
- Potassium Iodate
- Calcium Iodate
- Iodic acid
- Periodic acid
- Hydriodic Acid
- Iodobenzene Diacetate
- Dess Martin Periodinane
- 1,4-Diiodobenzene
- 4-Bromoiodobenzene
- 4-Chloroiodobenzene
- Iodobenzene
- 4-Iodoaniline
- 4-Iodotoluene
- 4-Iodoanisole
- 4-Iodophenol
- 4,4'-Diiodobiphenyl
- Trimethylsulfoxonium Iodide
Sodium Iodide
It is a Sodium salt of Hydriodic Acid [HI], available in white crystalline powder form, and has Molecular Formula NaI. Sodium Iodide is highly soluble in water. Sodium Iodide used generally as a reactant for conversion of alkyl chloride, or alkyl bromide into alkyl iodide in presence of Acetone [Finkelstein Reaction]. Due to its hygroscopic nature, it absorbs moisture rapidly from atmosphere. It is produced by treating Iodine with Sodium Hydroxide.
| Product | Sodium Iodide |
| Structural formula | 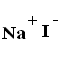 |
| CAS No. | [7681-82-5] |
| Molecular formula | NaI |
| Molecular weight | 149.89 |
| Description | White crystalline hygroscopic powder. |
| Iron | Max. 0.0050%. |
| Loss on drying | Max. 1.0%. |
| Assay | Between 99.00 % and 100.50 %. |