INVESTOR RELATIONS
- Methyl Iodide
- Ethyl Iodide
- n-Butyl Iodide
- N-propyl Iodide
- 2-Ethylhexyl Iodide
- Diiodomethane
- Chloroiodomethane
- 5-Iodo-2-methylbenzoic Acid
- 2-chloro-5-iodobenzoic acid
- Methyl-2-Iodobenzoate
- 2-Iodobenzoic Acid
- 3,5-Diiodosalicylic Acid
- N-Iodosuccinimide
- Tetrabutylammonium Iodide
- Iodine Monochloride
- 5-Iodoanthranilic acid
- Iodoform
- Potassium iodide
- Sodium Iodide
- Ammonium Iodide
- Cuprous Iodide
- Zinc Iodide
- Sodium Metaperiodate
- Potassium Iodate
- Calcium Iodate
- Iodic acid
- Periodic acid
- Hydriodic Acid
- Iodobenzene Diacetate
- Dess Martin Periodinane
- 1,4-Diiodobenzene
- 4-Bromoiodobenzene
- 4-Chloroiodobenzene
- Iodobenzene
- 4-Iodoaniline
- 4-Iodotoluene
- 4-Iodoanisole
- 4-Iodophenol
- 4,4'-Diiodobiphenyl
- Trimethylsulfoxonium Iodide
Iodic acid
Iodic acid, HIO3, can be obtained as a white solid. It dissolves in water very well, but it also exists in the pure state, as opposed to chloric acid or bromic Acid. Iodic Acid contains iodine in the oxidation state +5 and it is one of the most stable oxo-acids of the halogens in its pure state. When iodic acid is carefully heated, it dehydrates to iodine pentoxide. On subsequent heating, the iodine pentoxide further decomposes, giving a mix of iodine, oxygen and lower oxides of iodine.
| Product | Iodic Acid |
| Structural formula | 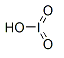 |
| CAS No. | [7782-68-5] |
| Molecular formula | HIO3 |
| Molecular weight | 175.91. |
| Description | Pale yellow crystalline powder. |
| Assay | Min 98.00% |